Written By: Raja R
In the realm of regulations, there is an increasing emphasis on the use of real-world evidence (RWE) to inform decision-making processes. RWE refers to data collected from real-world settings, such as electronic health records, claims databases, and patient registries, which can provide valuable insights into the safety, effectiveness, and value of medical interventions. This shift towards RWE is driven by the recognition that traditional clinical trials may not always capture the full spectrum of patient experiences and outcomes. By incorporating RWE into regulatory decision-making, regulators can make more informed and evidence-based decisions that reflect the real-world effectiveness and safety of interventions. This article explores the importance of sustaining focus on RWE in regulations and the benefits it brings to both regulators and stakeholders in the healthcare ecosystem.
Table of Contents
Importance of Real-world Evidence in Regulations:
Real-world evidence (RWE) serves as a vital compass for ensuring patient safety and enhancing healthcare efficacy. Unlike meticulously controlled clinical tests, RWE offers a real-world perspective, unveiling the actual experiences of doctors and patients. This valuable insight refines the rules governing medicines, providing a nuanced understanding of how diverse individuals are affected. The significance of RWE lies in its capacity to move beyond the binary assessment of whether medicines work and are safe. By tapping into the day-to-day experiences within healthcare settings, RWE delves into the practical, real-world impact of medicines on people’s lives. This goes beyond the controlled environment of clinical trials, offering a comprehensive understanding of how different demographics respond to treatments.
In essence, RWE is vital for informed decision-making in healthcare. It ensures that regulatory decisions and medical interventions are not solely based on efficacy and safety but are also attuned to the varied needs and realities of real-world patient populations. By safeguarding patient safety, optimizing treatment effectiveness, and contributing to a well-rounded healthcare plan, RWE emerges as a cornerstone in fostering a patient-centric and evidence-based approach to healthcare decision-making.
Challenges/Problems faced while implementing Real-world Evidence in Regulations:
Leveraging real-world evidence (RWE) in healthcare regulations presents a host of intricate challenges requiring meticulous consideration.
- A primary concern lies in the heterogeneous and often non-standardized nature of real-world data sources. Unlike the controlled setting of clinical trials, real-world data emanates from diverse origins, encompassing electronic health records, claims databases, and patient registries. The diversity in these sources poses a challenge in terms of data quality and standardization. Ensuring the reliability, consistency, and comparability of data across different settings is a substantial hurdle demanding strategic solutions.
- Another critical challenge revolves around inherent biases and confounding factors embedded in real-world data. Due to the varied and uncontrolled environments where this data is collected, it may be subject to influences such as patient demographics, socioeconomic status, and healthcare provider practices. The identification and mitigation of these biases are imperative to extract meaningful and accurate insights from real-world evidence. Regulatory harmonization adds a layer of complexity. Establishing a standardized approach to integrating real-world evidence across diverse global regulatory bodies is a multifaceted task. The establishment of consistent standards, methodologies, and acceptance criteria is crucial to ensure the credibility and uniform application of real-world evidence in regulatory decision-making.
Request a free proposal to understand how.
Success Story:
Driving Compliance: How Quantzig Enabled Real-world Evidence Focus for Regulatory Success
Client location:
USA
Challenges faced by the client:
- Our US-based client invested billions in researching and conducting clinical trials for a drug targeting a rare disease. However, getting regulatory approvals took much longer than expected, causing costs to soar and delaying the drug’s potential launch. The drug faced several hurdles in the approval process, leading to delays in obtaining FDA approval.
- The client urgently sought a way to expedite the approval process to reduce costs and bring the drug to market sooner. The extended waiting period and regulatory roadblocks were not only impacting the client’s budget but also hindering the timely availability of a crucial drug for patients in need.
- To address this, we proposed streamlined strategies and collaborative efforts with regulatory authorities to fast-track the approval process, ensuring the client’s investment in research and trials translates into timely and accessible healthcare solutions.
Solutions offered by QZ:
Quantzig effectively addressed the client’s challenges by harnessing the power of Real-World Evidence (RWE) data. Our experienced team conducted a thorough analysis of the RWE data, confirming the drug’s clinical benefits relative to existing market drugs. This comprehensive evaluation not only substantiated the drug’s efficacy but also provided compelling evidence to justify expedited regulatory approval. The strategic use of RWE insights empowered the client to present a robust case, enhancing their chances of obtaining timely regulatory approval and expediting the drug’s market launch. By relying on Quantzig’s expertise in RWE analytics, the client gained a strategic advantage, ensuring that their investment in research and trials translated into a swift and impactful market presence.
Impact Delivered
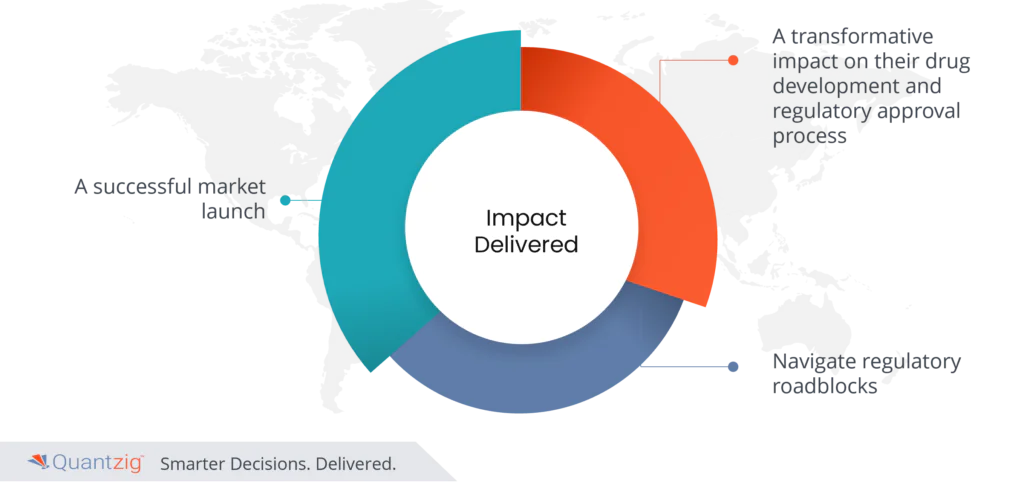
- Engaging with Quantzig, the client experienced a transformative impact on their drug development and regulatory approval process. Quantzig’s strategic analysis of Real-World Evidence (RWE) data played a pivotal role in confirming the drug’s clinical benefits compared to existing market drugs. This, in turn, strengthened the client’s case for expedited regulatory approval.
- The insights derived from the RWE data not only streamlined the approval process but also positioned the client for a successful market launch.
- The impact delivered included a more compelling and evidence-based regulatory submission, reducing the time and costs associated with prolonged approval timelines. Quantzig’s expertise in RWE analytics empowered the client to navigate regulatory roadblocks efficiently, ensuring a faster path to market and, ultimately, improving accessibility to a crucial drug for patients with rare diseases.
In conclusion, maintaining a sustained focus on real-world evidence (RWE) in regulations is crucial for ensuring the safety, efficacy, and quality of products and services. RWE provides valuable insights into the real-world performance of interventions, allowing regulatory bodies to make informed decisions based on actual outcomes and patient experiences.
By incorporating RWE into regulatory frameworks, policymakers can enhance the efficiency and effectiveness of regulatory processes. RWE enables the evaluation of long-term safety and effectiveness, identification of potential risks, and assessment of the impact of interventions on different patient populations. This approach promotes evidence-based decision-making, fosters innovation, and facilitates timely access to new treatments and technologies.
Furthermore, a sustained focus on RWE encourages collaboration between regulators, healthcare providers, industry stakeholders, and patients. This collaboration promotes the generation and utilization of high-quality RWE, leading to more robust regulatory decisions and improved patient outcomes.
As the healthcare landscape continues to evolve, it is imperative for regulatory bodies to adapt and embrace RWE as an integral part of their decision-making processes. By doing so, they can ensure that regulations are evidence-based, patient-centered, and responsive to the needs of the population. Ultimately, sustaining focus on RWE in regulations will contribute to the advancement of healthcare and the well-being of individuals worldwide.
Explore how Quantzig’s expertise can help your business sustain focus on real-world evidence. Contact us today for a consultation!