Written By: Sudeshna Ghosh
Table of Contents
Introduction to Pharma Real World Evidence
In the ever-evolving landscape of pharmaceuticals, there is a palpable buzz surrounding the concept of “real-world evidence” (RWE). Gone are the days when drug development and approval relied solely on tightly controlled clinical trials. Instead, the industry is increasingly turning to the vast reservoir of data generated in real-world settings to inform and revolutionize decision-making. Pharma’s fascination with RWE represents a seismic shift, where the quest for safer, more effective medicines is no longer confined to the laboratory, but extends into the realm of everyday patient experiences. This paradigm shift promises to unlock new frontiers in drug development, regulatory approval, and patient-centric healthcare, making RWE the focal point of innovation and conversation within the pharmaceutical world.
Book a demo to experience the meaningful insights we derive from data through our GenAI tools and platform capabilities. Schedule a demo today!
Request a Free DemoWhat is Pharma Real-world Evidence (RWE)?

Pharma real-world evidence (RWE) is a valuable tool that complements data generated from randomized controlled trials (RCTs). It provides insights into how medications are used in routine clinical practice, helping to identify potential patients and inform pre-trial study design and post-market surveillance. RWE also improves pharmacovigilance, strengthens evidence for differentiation, and enhances adherence and patient-reported outcomes. By leveraging RWE, pharmaceutical companies can unlock significant financial benefits, improve healthcare decision-making, and drive clinical and commercial objectives.
Importance of Pharma Real World Evidence
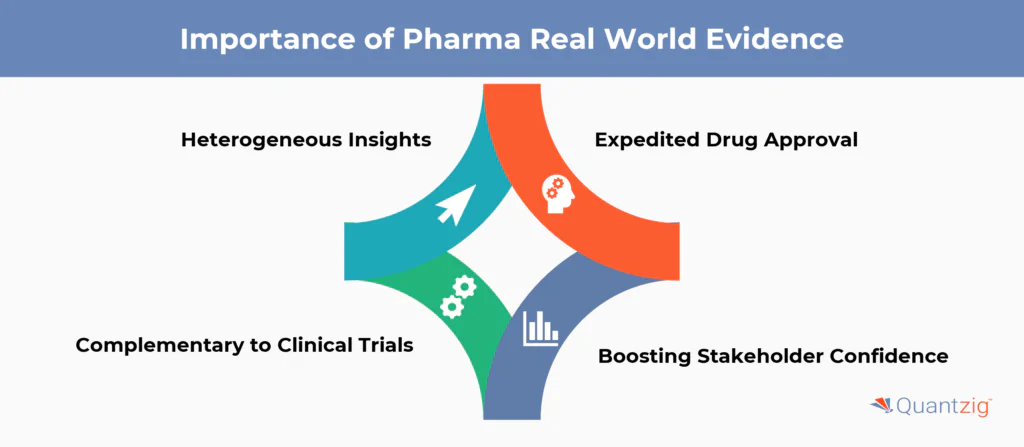
- Heterogeneous Insights: RWE is heterogenous in nature unlike controlled clinical trial data and can provide deeper insights about how heterogenous real-world population will respond to a drug/treatment. Thus, any insights from RWE are lot closer to actual patient population reaction.
- Expedited Drug Approval: Regulators take RWE into account during the approval process and hence pharma companies can use RWE to their advantage to expedite drug approval.
- Complementary to Clinical Trials: RWE complements clinical trial data by offering insights that trials alone cannot provide, enriching the understanding of a drug’s real-world impact.
- Boosting Stakeholder Confidence: RWE can help bolster confidence of insurance companies and other stakeholders which ultimate impacts the drugs perception and revenue uptake.
What is Driving the Interest in Real-World Evidence in Pharma?
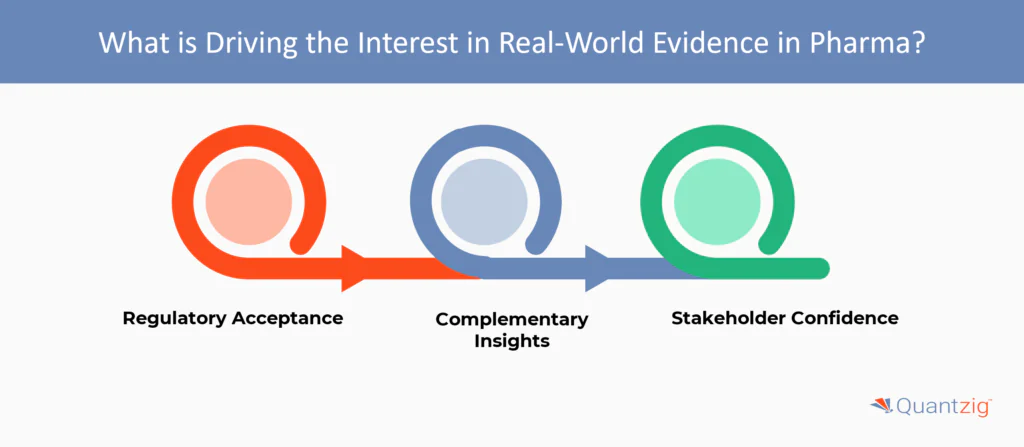
The pharmaceutical industry is increasingly drawn to real-world evidence (RWE) due to several key factors. These include:
- Regulatory Acceptance: RWE has the potential to influence regulatory approvals and accelerate the drug development process, which could significantly reduce costs for drugmakers.
- Complementary Insights: RWE complements clinical trial data by providing valuable insights into safety and effectiveness in real-world settings. This is particularly important as clinical trials often have narrow eligibility criteria and intent-to-treat analyses, which can lead to surprising findings when the constraints are set aside.
- Stakeholder Confidence: Insurance companies and other stakeholders require a solid understanding of a drug’s benefits for patients. RWE can bolster their confidence in a treatment’s effectiveness by providing evidence of improved patient outcomes and reduced hospitalization rates and lengths of stay. According to Quantzig experts, “Insurance companies, particularly in the US, are looking for medicines that can demonstrate improved patient outcomes and reduce hospitalization rates and length of stays”.
These factors collectively contribute to the growing interest in real-world evidence in the pharmaceutical industry, as it is a powerful tool for informing decision-making and improving healthcare outcomes.
Challenges While Implementing Pharma Real World Evidence
- Diverse Data Sources: Multiple RWE datasets capture different types and levels of information, making it challenging to analyze and extract coherent insights across all sources.
- Regional Data Variability: Regional GDPR rules create data disparities across geographies, adding barriers to insight identification and analysis.
- Complex Analysis: RWE data analysis is complex, time-consuming, and costly due to the scarcity of resources with comprehensive knowledge of various RWE datasets and their interpretive nuances.
What are the Benefits of Pharma Real World Evidence?
1. Boost drug development and approval process:
Pharma’s growing enthusiasm for real-world evidence (RWE) heralds a transformational era in drug development and approval, offering a multitude of benefits across the pharmaceutical value supply chain. Firstly, RWE accelerates and refines the drug development process by leveraging insights from diverse, real-world patient populations. By analyzing data from uncontrolled settings, it provides a more comprehensive understanding of a drug’s performance, efficacy, and safety, ultimately streamlining regulatory approval.
2. Boost confidence of stakeholders across pharma value chain:
Moreover, RWE instills greater confidence among stakeholders, including pharmaceutical companies, healthcare providers, and regulators. This heightened confidence arises from the fact that RWE draws from the experiences of actual patients, making it closer to real-world scenarios. As a result, decision-makers can make more informed choices regarding treatment options, pricing strategies, and healthcare policies.
3. Insights from heterogenous data across uncontrolled patient population:
RWE taps into a diverse and uncontrolled patient population, providing insights that closely mirror real-world scenarios. This approach enhances drug development and regulatory decision-making by offering a more accurate understanding of a drug’s effectiveness and safety profile.
Get started with your complimentary trial today and delve into our platform without any obligations. Explore our wide range of customized, data driven analytical solutions built across the analytical maturity levels.
Start your Free TrialHow does RWE Help Across the Pharma Value chain?
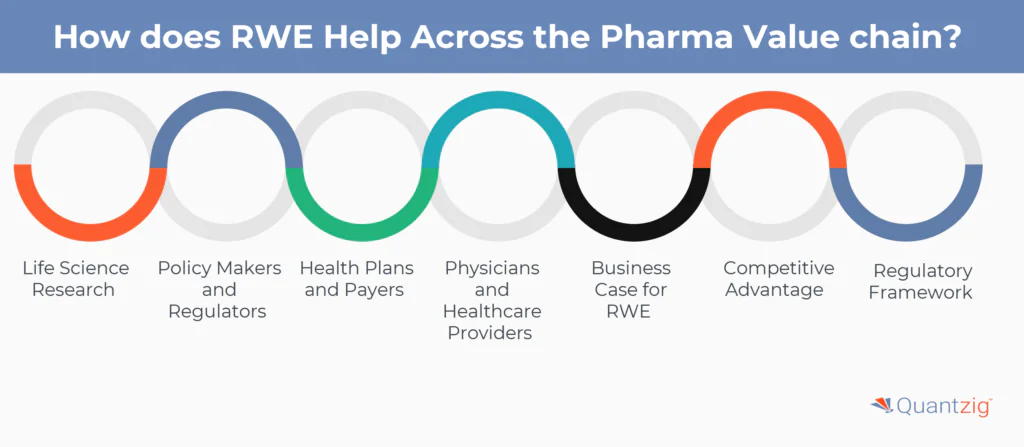
Real-world evidence (RWE) plays a critical role across the pharma value chain by enhancing every stage from drug development to post-market surveillance. By utilizing real-world data (RWD) such as electronic health records (EHRs), disease and product registries, claims and billing data, and pharmacy claims data, pharmaceutical companies can gain comprehensive RWE insights that complement traditional clinical trials. These insights provide drugmakers with crucial safety and effectiveness data, helping to optimize observational trials and support regulatory submissions. In the RWE market, leveraging this data enables pharmaceutical companies to demonstrate the real-world impact of their products, leading to improved patient outcomes and informed decision-making throughout the product lifecycle.
1. Life Science Research
Life science researchers and biopharmaceutical companies utilize RWE to gain additional insights into medication use in routine clinical practice, thereby informing the safety and efficacy of investigated products. RWE also helps identify potential patients and create proper inclusion criteria for pre-trial study design and post-market surveillance.
2. Policy Makers and Regulators
RWE helps improve evidence of economic value by demonstrating the value of treatment to payers, comparing RCT data with RWE data to complement the dossier, and enabling outcomes-based pricing. This enhances the regulatory framework, allowing for more informed decision-making.
3. Health Plans and Payers
RWE provides valuable insights from real-world settings, complementing data generated from randomized controlled trials (RCTs). Payers increasingly rely on RWE for greater validity in allocating resources, which can lead to value-based care outcomes.
4. Physicians and Healthcare Providers
RWE helps improve pharmacovigilance through monitoring the safety profile of health technology, strengthens evidence for differentiation by identifying subgroups for which effect outperforms trials, and improves adherence and patient-reported outcomes (PROs) with digital tools. This enhances the overall quality of healthcare delivery.
5. Business Case for RWE
Pharmaceutical companies can leverage RWE to drive clinical and commercial objectives, providing greater clarity for healthcare providers and improved access to therapy for patients. RWE helps address critical clinical and safety questions that arise between the end of RCTs and longer-term use of drugs in day-to-day clinical practice. By analyzing real-world data (RWD) from various sources, drug manufacturers can validate treatment performance in heterogeneous patient populations, demonstrating safety and efficacy in subgroups not included in RCTs.
6. Competitive Advantage
Pharmaceutical companies can unlock significant financial benefits by adopting advanced RWE analytics across their value chain. McKinsey & Company estimates that top-20 pharma companies could unlock over $300 million annually over three to five years by leveraging RWE. Additionally, RWE vendors are investing in sophisticated analytics platforms, AI, and NLP technologies to deliver insights across the drug development, care delivery, and commercial life cycles.
7. Regulatory Framework
Evolving regulatory precedents and frameworks signal increased receptivity among regulators, creating momentum for pharmaceutical companies to use RWE-based initiatives. Regulatory bodies such as the US Food and Drug Administration (FDA) and the UK’s National Institute for Health and Care Excellence (NICE) are encouraging the use of RWE for various applications, including safety and effectiveness evaluations, and supporting submissions and approvals.
The RWE market is expected to grow rapidly, driven by the increasing availability and integration of health data. Pharmaceutical companies should consider hiring personnel with experience in using RWE insights to improve business decisions and leverage advisory service offerings from RWE vendors for specific areas where in-house talent may be lacking.
Quantzig’s Expertise in Pharma Real World Evidence Solution for a Leading Pharmaceutical Company
| Category | Details |
|---|---|
| Client Details | A leading pharmaceutical company located in the USA, renowned for its diverse product portfolio and widespread presence. |
| Challenges Faced by The Client | – Increased non-adherence to treatment protocols – Struggled to identify reasons behind non-adherence |
| Solutions Offered by Quantzig | – Automated Life Stage-Value Prediction Model – Patient Claims Data and Administrative Claims Data – Benchmarked patients across similar cohorts – Visualized data for stakeholders |
| Impact Delivered | – Improved patient care through insights into patient life stage value and cost drivers – Increased patient adherence and reduced healthcare costs – Enhanced business outcomes through improved patient care – Competitive advantage through data-driven insights |
Client Details:
A leading pharmaceutical company located in the USA.
The challenges faced by the Client:
The client was witnessing increased non-adherence to treatment protocols among the patient population and struggled to identify the reasons behind it. A competing drug was gaining market share while their own product faced challenges. The client sought to analyze the root causes of this issue.
Solutions offered by Quantzig:
Quantzig built an automated, life stage-value prediction model capable of determining the cost drivers for patients in the highest healthcare cost cohort. Patient claims data and administrative claims data were used to benchmark patients across similar cohorts to identify segments with high costs and low outcomes. The solution was visualized for stakeholders to easily identify and explore individual patient opportunities, ultimately improving patient care by providing insights into patient life stage value and cost drivers.
Impact Delivered:
- Improved patient care through insights into patient life stage value and cost drivers
- Increased patient adherence and reduced healthcare costs
- Enhanced business outcomes through improved patient care
- Competitive advantage through data-driven insights
Experience the advantages firsthand by testing a customized complimentary pilot designed to address your specific requirements. Pilot studies are non-committal in nature.
Request a Free PilotThe Future of Real-world Data and Real-world Evidence in Pharma

The future of real-world data (RWD) and real-world evidence (RWE) in pharma is promising, driven by the growing importance of embracing and understanding RWD. The COVID-19 pandemic has accelerated the adoption of RWD and RWE in drug development, with projects like the COVID-19 Evidence Accelerator demonstrating the potential of RWE in advancing research. Key factors contributing to the excitement about RWE include its potential to impact regulatory approvals, accelerate the drug development process, and provide insights that clinical trials cannot.
Additionally, RWE complements clinical trials data by offering safety and effectiveness data from a patient’s daily life, which is valuable for insurance companies and other stakeholders. As the use of RWE expands, it is crucial to address data-quality challenges and ensure that RWD is generated in a patient-centric way, respecting patients’ rights to privacy and data ownership.
Conclusion
The pharmaceutical industry‘s growing focus on real-world evidence represents a transformative shift towards more patient-centric, data-driven decision-making. By leveraging the heterogeneity of RWE, overcoming data challenges, and embracing complex analysis, pharmaceutical companies are poised to enhance drug development, accelerate approvals, and bolster stakeholder confidence. In an era where patient outcomes are paramount, RWE is paving the way for safer, more effective pharmaceuticals.