Table of Contents
Table of Contents
- Introduction
- Top Medical Devices Industry Challenges
- Key Strategies to Address Medical Devices Industry Challenges
- Quantzig’s Solution to solve Medical Devices Industry Challenges
- Conclusion
Author: Associate Vice President, Analytics and Data Strategy, Quantzig.
Introduction to Medical Devices Industry Challenges
The medical device industry has faced significant changes in recent years, driven by the shift towards value-based care and reimbursement models. Focusing solely on data and the value of medical devices is no longer sufficient to drive profitability. Companies in this sector must explore new revenue streams and strategies to adapt to the evolving landscape. The transition to value-based care requires solutions that can provide actionable insights seamlessly integrated into existing electronic medical record systems. This shift has created several critical challenges that are putting pressure on the medical device industry to innovate and adapt their business models to remain competitive and profitable in the new healthcare environment.
Book a demo to experience the meaningful insights we derive from data through our analytical tools and platform capabilities. Schedule a demo today!
Request a Free DemoWhat are the Top Medical Devices Industry Challenges?
The competition in the medical device industry is soaring to a whole new level, prompting players in this space to manufacture more complex and integrated products to stay ahead of their peers in the market. Consequently, this results in a rise in the cost of field service and repair processes. Moreover, this has also doubled the cost of training field service specialists and engineers. The medical devices industry faces a multitude of challenges spanning cybersecurity threats to interconnected devices, regulatory demands from agencies like the FDA ensuring compliance, and the need for robust supply chain resilience amid raw material shortages. Quantzig’ s cost analytics solutions help medical devices companies identify and set the optimum price for their products and yield maximum ROI.
1. Meeting customer expectations
The advent of technology has given customers around the world access to the internet and smartphones. They now have numerous choices at their disposal and demand more from companies in the medical device industry, especially in terms of implementing more aggressive SLAs. The end-users of medical devices are demanding more incentives from their reduces capital.
2. Shift towards home-based care
The popularity of home healthcare services and POC tests are skyrocketing, this means that players in the medical device industry need to follow suit and migrate towards the production of home-based health kits. This would pose a threat in reducing the spending on capital programs for the development of new lab equipment.
3. Cut-throat competition
Manufacturers in the medical device industry are gradually losing their ability to fund capital for their new devices primarily due to the implementation of new schemes to reduce reimbursements for medical assays by insurers. To add on, heightening competition from other emerging companies in the market will contribute to eroding customer loyalty and thereby reducing the profit margins of top medical device companies.
4. Ongoing Supply Chain Issues
The medical device industry has faced persistent supply chain problems since the pandemic, including rising raw material prices, increased demand, and disrupted distribution networks. Companies must be agile and leverage data intelligence to adapt to these supply chain challenges.
5. More Regulatory Changes
The pandemic led to regulatory changes around medical devices, some of which may present opportunities for future product development and approval. However, the industry must navigate this evolving regulatory landscape.
6. Demand for Faster Product Development
The pandemic accelerated the timeline for medical device development, putting pressure on companies to balance these new expectations with their traditional research and development processes. Leveraging technology and agility is key to meeting this demand.
7. Quality Concerns and Product Recalls
Poor quality in medical devices can lead to patient injuries and costly product recalls, which can significantly damage a company’s reputation and finances. Maintaining rigorous quality control is critical.
8. Counterfeiting
The rise of counterfeit medical devices poses a serious threat to patient safety. Anti-counterfeiting technologies are helping the industry combat this challenge.
9. Regulatory Compliance
The highly regulated nature of the medical device industry means companies must navigate complex and varying regulatory requirements across different markets, which can be a significant burden.
10. Cybersecurity
As medical devices become more connected, the risk of cyberattacks on healthcare systems increases. Manufacturers must ensure their devices are equipped with robust cybersecurity measures.
11. Inefficient Business Processes
Outdated or siloed business processes can hinder medical device manufacturers’ ability to ensure quality, traceability, and compliance. Adopting modern ERP systems can help streamline operations.
Addressing these challenges requires a strategic approach that integrates advanced technology solutions with in-depth attention to regulatory requirements, fostering collaboration across stakeholders to ensure patient safety and industry sustainability.
Also Read: Pharma Customer Segmentation Helped a Client Improve Customer Satisfaction by 10%
Experience the advantages firsthand by testing a customized complimentary pilot designed to address your specific requirements. Pilot studies are non-committal in nature.
Request a Free PilotKey Strategies to Address Medical Devices Industry Challenges
The medical devices industry faces several challenges, and addressing them requires a combination of innovative approaches, regulatory compliance, and strategic planning. Here are some key strategies to tackle these challenges:
| Challenge | Strategy |
|---|---|
| Regulatory Compliance | – Stay updated with regulations like FDA, CE marking, and other relevant standards – Ensure all products meet regulatory requirements to avoid delays and penalties |
| Quality Assurance | – Implement robust quality management systems (QMS) – Maintain high standards of product safety and efficacy through rigorous testing, documentation, and adherence to Good Manufacturing Practices (GMP) |
| Innovation and R&D | – Invest in research and development to foster innovation – Develop new technologies that improve patient outcomes, reduce costs, or enhance usability |
| Supply Chain Management | – Optimize supply chain processes to ensure timely delivery of components and materials – Build resilience to disruptions and maintain a secure supply chain |
| Cybersecurity | – Prioritize cybersecurity measures to protect against data breaches and ensure patient privacy and safety |
| Market Access and Pricing | – Navigate reimbursement challenges and pricing pressures – Demonstrate the value of devices through clinical evidence and cost-effectiveness studies |
| Global Market Expansion | – Understand regulatory and cultural differences when expanding into international markets – Adapt products and strategies to meet local requirements and preferences |
| Lifecycle Management | – Manage the entire product lifecycle effectively, from development and launch to post-market surveillance and eventual phase-out or replacement |
| Collaboration and Partnerships | – Foster collaborations with healthcare providers, researchers, and technology partners to leverage expertise and resources for mutual benefit |
| Patient-Centric Approach | – Focus on patient needs and preferences in device design and usability – Engage with patients and healthcare professionals for feedback and insights |
By addressing these challenges in medical device industry proactively and strategically, companies in the medical devices industry can enhance their competitive position, ensure compliance, and deliver value to patients and healthcare providers.
How Can Quantzig Help to Solve Medical Devices Industry Challenges?
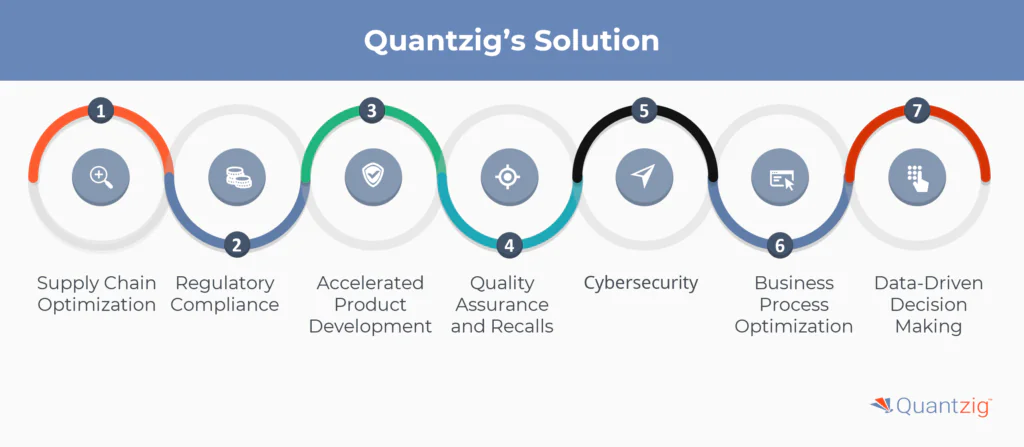
Quantzig can help the medical device industry address several key challenges:
1. Supply Chain Optimization
We offer advanced analytics solutions to help medical device companies navigate supply chain disruptions. This includes demand forecasting, inventory optimization, and distribution network analysis to build more resilient and agile supply chains.
2. Regulatory Compliance
Quantzig’s expertise in regulatory analytics can assist medical device manufacturers in navigating the evolving regulatory landscape. This includes solutions for quality control, product traceability, and post-market surveillance to ensure compliance.
3. Accelerated Product Development
Our team leverages data-driven insights and process automation to help medical device companies streamline their R&D and product launch timelines. This includes solutions for clinical trial optimization, predictive failure analysis, and integrated product development workflows.
4. Quality Assurance and Recalls
Our advanced analytics capabilities can help identify quality issues early and mitigate the risk of product recalls. This includes real-time quality monitoring, predictive maintenance, and root cause analysis solutions.
5. Cybersecurity
We offer specialized services to assess and enhance the cybersecurity posture of connected medical devices. This includes vulnerability assessments, threat modeling, and the implementation of robust security controls.
6. Business Process Optimization
Our expertise in business process automation and ERP integration can help medical device manufacturers streamline their operations, improve traceability, and drive greater efficiency across the organization.
7. Data-Driven Decision Making
Our advanced analytics and data visualization solutions empower medical device companies to make more informed, data-driven decisions across their product lifecycle, from R&D to commercialization and post-market surveillance.
By leveraging Quantzig’s comprehensive suite of analytics services, medical device companies can navigate the industry’s challenges, improve operational efficiency, ensure regulatory compliance, and drive sustainable growth in the evolving healthcare landscape.
Also Read: Customer Loyalty Analysis for a Leading Pharma Player in Europe
Get started with your complimentary trial today and delve into our platform without any obligations. Explore our wide range of customized, consumption driven analytical solutions services built across the analytical maturity levels.
Start your Free Trial TodayConclusion
The medical device industry faces numerous challenges, including cybersecurity, FDA regulatory requirements, and supply chain resilience amid raw material shortages. Medtech companies and OEMs must invest in R&D and adopt additive manufacturing and smart manufacturing to enhance production efficiency and reduce costs. Ensuring robust cybersecurity, compliance with regulatory demands, and maintaining a resilient supply chain are essential. By leveraging advanced technology and innovative solutions, medical device manufacturers can overcome these obstacles and continue delivering high-quality, cutting-edge products to support frontline healthcare and meet evolving market needs.