Table of Contents
Table of Contents
- Introduction
- What is AI Pharmacovigilance?
- Role of AI in Pharmacovigilance
- Applications of AI in Pharmacovigilance
- Forms of AI in Pharmacovigilance
- Challenges and Limitations of AI in Pharmacovigilance
- Conclusion
Author: Associate Vice President, Analytics and Data Strategy, Quantzig.
Introduction to AI Pharmacovigilance
Greater emphasis on drug safety is one of the key drivers of the growing demand for pharmacovigilance (PV) software. With the focus on all the major drug regulations being on improving the drug quality and prevention of drug-related problems, pharma companies are increasingly becoming dependent on PV tools. However, the task becomes rather difficult in situations where huge amounts of patient data are being generated on a regular basis. Consequently, a major pain point for most pharma companies face is the execution of foolproof digital vigilance on the data generated.
The lack of efficient and coordinated systems that can seamlessly integrate various data into actionable insights is a concern for most in the pharma industry. It is in this context that artificial intelligence (AI) comes into play. By employing data mining and taking electronic health records (EHRs) into consideration, AI facilitates PV to provide better medication management and improved health assistance.
Book a demo to experience the meaningful insights we derive from data through our analytical tools and platform capabilities. Schedule a demo today!
Request a Free DemoWhat is AI Pharmacovigilance?
The integration of Artificial Intelligence (AI) into pharmacovigilance has emerged as a transformative force, revolutionizing the monitoring and assessment of drug safety. AI-driven algorithms, machine learning, and natural language processing empower automated signal detection, enabling more efficient and proactive risk assessment. The use of AI can help reduce human error and speed up the process of risk assessment by analyzing large amounts of data to identify patterns and trends.
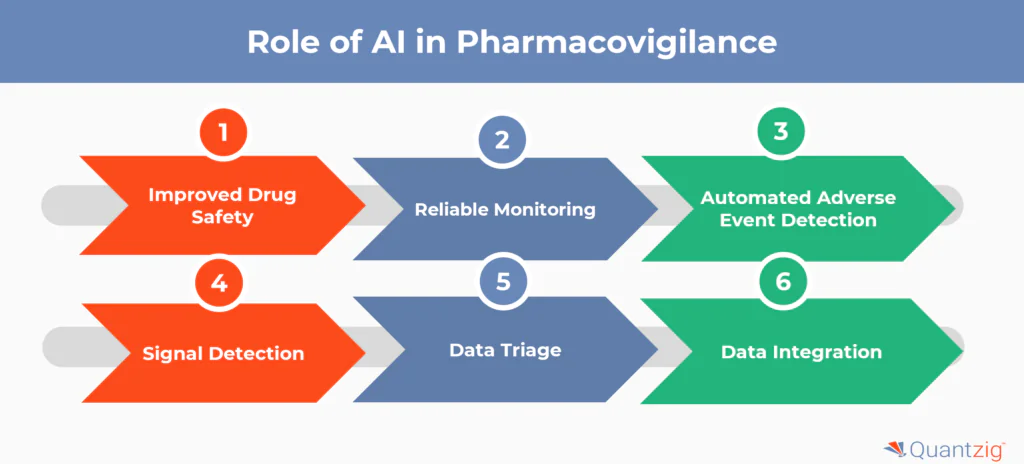
What is the Role of AI in Pharmacovigilance?
1. Improved Drug Safety
- AI can easily decipher the natural human semantics, which means that the chances of blind searches are almost negligible.
- By straining the irrelevant information and taking the subtext of the data into consideration, AI provides a focused analysis of drug use and patient requirement.
- As a result, all the integral side-effects of drugs are considered so that the safety of the patients is not compromised at any cost.
2. Reliable Monitoring
- The whole point of adopting PV is to bring reliable monitoring into the pharma industry. As the internet never sleeps, it becomes even more critical for companies to keep a 24×7 digital vigilance.
- Not only is AI-based web monitoring better than human supervised ones; its preloaded algorithm also makes it easier for pharma firms to track the online data holistically rather than focusing on bits and pieces.
3. Automated Adverse Event Detection
- AI, particularly Natural Language Processing (NLP), can automatically identify and extract information about adverse events from large datasets like electronic health records, social media, and medical literature. This allows for faster and more comprehensive detection of potential safety issues.
4. Signal Detection
- Machine learning algorithms can analyze data to identify potential safety signals and patterns that may not be easily detected manually. AI-powered signal detection helps identify emerging drug safety concerns more efficiently.
5. Data Triage
- AI can help prioritize adverse event reports based on potential severity, allowing pharmacovigilance professionals to focus on high-priority cases that require immediate attention. This improves the efficiency of case processing and resource allocation.
6. Data Integration
- AI can integrate data from multiple sources, such as clinical trials, post-marketing surveillance, and real-world evidence, to provide a more comprehensive view of drug safety. By combining data from various sources, AI enables a holistic assessment of a drug’s safety profile.
Book a demo to experience the meaningful insights we derive from data through our analytical tools and platform capabilities. Schedule a demo today!
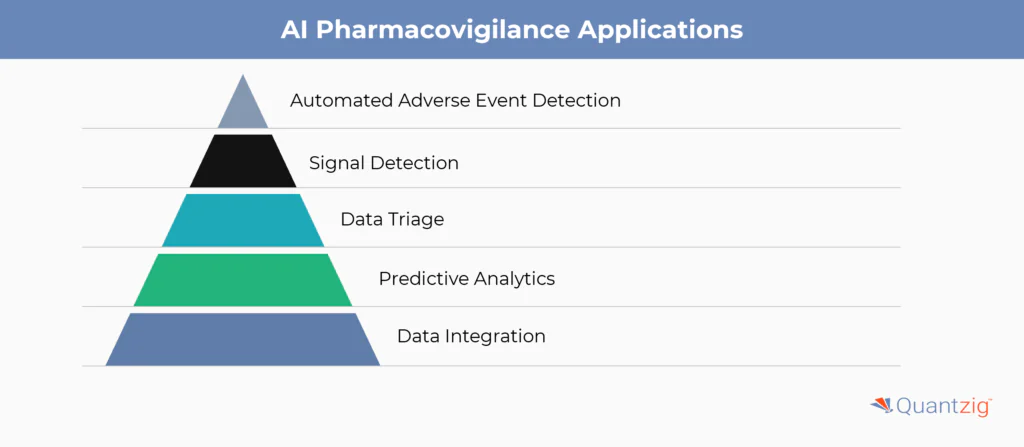
Book a Free DemoApplications of AI in Pharmacovigilance
- Automated Adverse Event Detection: AI, particularly Natural Language Processing (NLP), can automatically identify and extract information about adverse events from large datasets like electronic health records, social media, and medical literature.
- Signal Detection: Machine learning algorithms can analyze data to identify potential safety signals and patterns that may not be easily detected manually.
- Data Triage: AI can help prioritize adverse event reports based on potential severity, allowing pharmacovigilance professionals to focus on high-priority cases.
- Predictive Analytics: Machine learning models can predict the likelihood of adverse events based on patient data, drug usage, and other factors, enabling proactive management of drug safety.
- Data Integration: AI can integrate data from multiple sources to provide a more comprehensive view of drug safety.
Forms of AI in Pharmacovigilance
- Machine Learning: Algorithms that learn from data and make predictions or decisions without being explicitly programmed.
- Natural Language Processing (NLP): Enables computers to analyze, understand, and generate human language, allowing for automated processing of unstructured data like medical literature and social media posts.
- Deep Learning: A type of machine learning that uses artificial neural networks with multiple layers to learn from large datasets and make predictions.
Challenges and Limitations of AI in Pharmacovigilance
- Data Quality: Ensuring the quality, completeness, and accuracy of data used to train AI models is crucial for reliable outputs.
- Interpretability: Explaining the decision-making process of complex AI algorithms can be challenging, which is important for regulatory compliance and trust in the technology.
- Regulatory Concerns: Integrating AI into pharmacovigilance workflows requires addressing regulatory issues around data privacy, algorithm validation, and transparency.
- Technical Malfunctions: AI systems can malfunction, and organizations must be prepared to troubleshoot findings and resolve flaws in the algorithms.
- Training of Drug Safety Professionals: Increased effort and time must be invested to train relevant personnel on the use and implications of AI technologies in pharmacovigilance.
AI has significant potential to enhance pharmacovigilance by improving the efficiency, accuracy, and speed of adverse event detection, signal identification, and case processing. However, addressing challenges related to data quality, interpretability, and regulatory compliance is crucial for the successful implementation of AI in this domain.
Conclusion
The role of Artificial Intelligence in enhancing pharmacovigilance is transformative for the pharmaceutical landscape. By leveraging advanced technologies, AI empowers drug safety professionals to efficiently monitor drug reactions and predict drug-induced repolarization disorders. This improves drug development processes, ensuring the safety of new drugs and therapeutics. AI mitigates risks of drug shortages and drug recalls, optimizes laboratory parameters, and enhances the health care system. Pharmaceutical companies benefit from reduced costs and adherence to global safety standards set by worldwide safety organizations. Thus, AI is crucial in ensuring safer medications and advancing the pharmaceutical industry.
Experience the advantages firsthand by testing a customized complimentary pilot designed to address your specific requirements. Pilot studies are non-committal in nature.
Request a Free Pilot