Table of Contents
Summary
Client: Quantzig, a leading analytics advisory firm, collaborated with a major pharmaceutical giant operating in the field of oncology. The client sought to improve nodal compliance adherence and decrease the likelihood of relapse among two distinct treatment groups, each comprising 250 patients. The objective was to enhance patient outcomes and optimize treatment strategies for melanoma.
Challenges: The pharmaceutical giant faced several challenges in its pursuit of improved nodal compliance adherence and reduced relapse rates. These challenges included limited insights into patient behavior, inadequate monitoring mechanisms, and the absence of a standardized framework to compare the outcomes of two distinct treatments. Overcoming these hurdles was crucial to improving patient care and increasing the efficacy of melanoma treatments. Besides, each geography, referring to different regions or countries, had its own unique set of compliance protocols that needed to be followed.
Solutions: Quantzig first developed a comprehensive framework aimed at establishing a standardized compliance protocol. To ensure the reliability of the patient data, the trial centers conducted ongoing quality control checks at regular intervals. Our team designed parametric hazard models, life tables, and competing risk methods to assess patient survival rates and disease recurrence rates in different groups.
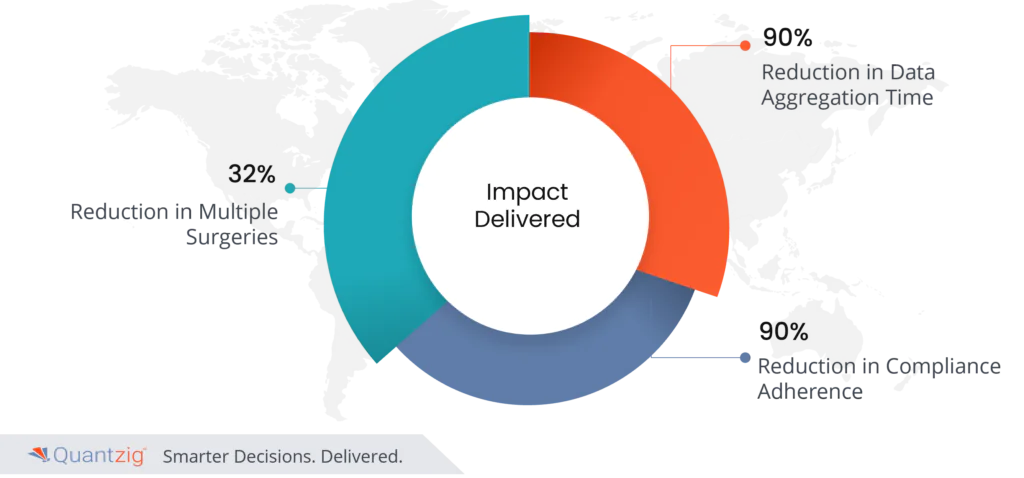
Impact Delivered
All of the above initiatives resulted in:
- 32% reduction in multiple surgeries
- 90% reduction in data aggregation time
- 90% reduction in compliance adherence
Industry Overview
- The USA multinational pharma industry plays a significant role in contributing to the country’s economic growth, and it is renowned for its innovation and research capabilities.
- Compliance analytics leverages advanced technologies, such as machine learning and data analytics, to monitor and analyze vast amounts of data related to regulatory compliance. It helps pharmaceutical companies identify and mitigate compliance risks, detect fraud, and misconduct, and enhance overall operational efficiency. Compliance analytics plays a crucial role in maintaining the industry’s reputation, ensuring patient safety, and fostering transparency in an ever-evolving regulatory landscape.
About the Client
- Our client, a multinational pharma giant based in the USA, operated in the rare disease market, particularly focusing on oncology. With annual revenue exceeding $2 billion, they sought to conduct a comparative analysis of disease recurrence rates and patient survival rates between two distinct patient groups. They needed to ensure strict adherence to the established procedure throughout the study. By comparing these rates, the client aimed to gain valuable insights into the effectiveness of their treatments and potentially identify factors that influence disease recurrence and patient outcomes, contributing to improved care for oncology patients in the future.
Challenges
- The client’s research project involved collaborating with over 10 hospitals worldwide, each employing different parameter tracking systems. This created a complex scenario when it came to comparing the results obtained from these diverse systems. With various hospitals employing different methods and technologies to track and record parameters, it became challenging to standardize the data for meaningful analysis and comparison. The disparities in data collection methods, formats, and measurement units hindered the ability to directly compare and draw conclusive findings. Overall, the client wanted to overcome the complexities arising from the differences in parameter tracking systems, focusing on standardization, data quality, and careful analysis to ensure meaningful and accurate research outcomes.
- Each geography, referring to different regions or countries, had its own unique set of compliance protocols that needed to be followed. These protocols encompassed various regulations, laws, and guidelines specific to each location. Adhering to these protocols was crucial to ensure legal and ethical compliance within each jurisdiction. International organizations need to carefully navigate and understand the intricacies of each region’s compliance requirements to avoid penalties, legal issues, or reputational damage. This involves staying updated on local legislation, data privacy laws, taxation regulations, labor laws, and other relevant guidelines to align their operations accordingly and maintain a consistent level of compliance across different geographies.
- The absence of a standardized framework for comparing the outcomes of two distinct treatments led to significant challenges for the company. Without such a framework, it became difficult to objectively evaluate the effectiveness of different treatment approaches and determine which one yields better results. This lack of uniformity impeded the progress of medical research, as it hampered the ability to draw meaningful conclusions from studies and compare findings across various treatment interventions. Additionally, the absence of a standardized framework hindered healthcare professionals’ ability to make informed decisions about the most appropriate treatment option for their patients, potentially leading to inconsistent and suboptimal care.
Solutions
- Quantzig developed a comprehensive framework aimed at establishing a standardized compliance protocol. This framework leveraged cutting-edge compliance analytics tools and techniques to ensure the company adheres to regulatory requirements consistently. Thus, we helped the client to proactively monitor and assess their compliance status, identify potential risks, and implement corrective actions promptly.
- To ensure the reliability of the patient data, the trial centers conducted ongoing quality control checks at regular intervals. These checks aimed to assess the completeness and accuracy of the collected data. However, despite these efforts, the pharmaceutical major expressed dissatisfaction with the results obtained during the initial analysis of the data. They were concerned about the level of precision and completeness in the data set. As a result, they decided to implement further measures to improve the quality control process and enhance the accuracy of patient data.
- Quantzig played a crucial role in assisting the client in designing parametric hazard models, life tables, and competing risk methods to assess patient survival rates and disease recurrence rates in different groups. Through our expertise in data analysis and statistical modeling, we applied these compliance analytics methods to the client’s dataset, incorporating relevant variables such as age, gender, and disease stage. By leveraging these advanced techniques, we were able to derive accurate estimates of survival rates and recurrence rates for each group, providing valuable insights for the client’s decision-making process. Our work helped the client understand the prognosis and risk factors associated with the disease, leading to improved patient care and more targeted interventions.


