Table of Contents
Summary
Client Details: The case study focuses on a multinational pharmaceutical giant headquartered in the USA with annual revenue of over $2 billion. The client operates in the rare disease market space and is dedicated to improving patient outcomes through innovative treatments. In this case, they sought to determine the chance of relapse of melanoma across two different groups consisting of 250 patients.
Challenges: The client needed to evaluate the risk of relapse for patients in two different treatment groups. They faced challenges in extracting meaningful insights from the available data due to the complex nature of melanoma and the diverse characteristics of the patient groups. They needed to leverage Clinical Trial Analysis combined with Digital Medical Affairs solutions to obtain comprehensive and reliable insights from the data.
Solutions:
Data collection and cleansing: Quantzig collaborated with the client to collect and cleanse the patient data, ensuring its accuracy, completeness, and consistency. Data from the two treatment groups were compiled, organized, and standardized for further analysis.
Statistical modeling: Our team designed parametric hazard models and employed clinical trial analysis techniques to assess the relapse probability for melanoma patients in each treatment group. These models considered various risk factors and predictors, enabling a comprehensive analysis of relapse chances.
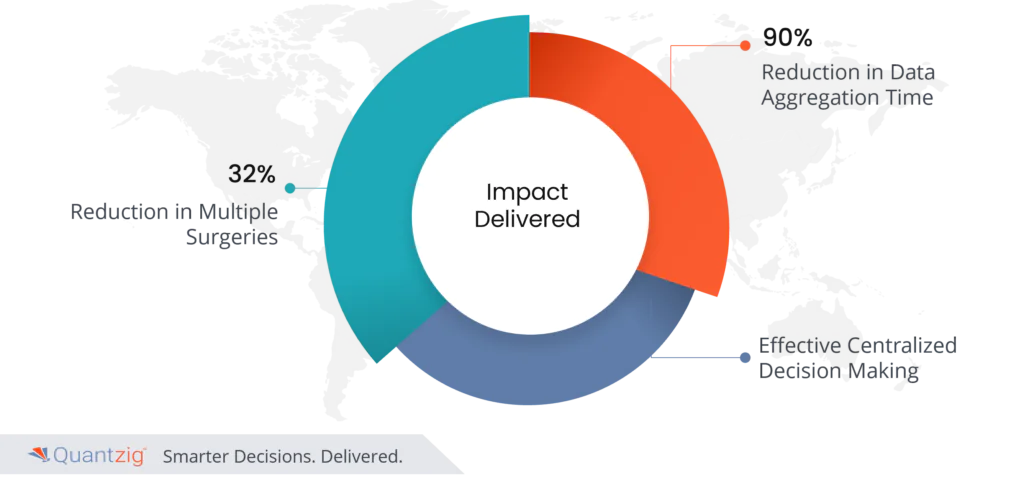
Impact Delivered
All of the above initiatives resulted in:
- 32% reduction in multiple surgeries
- 90% reduction in data aggregation time
- Effective centralized decision-making
Industry Overview
- The USA pharmaceutical industry continues to be a dynamic and influential sector, driving innovation, research, and development, while aiming to improve healthcare outcomes and enhance the quality of life for patients worldwide.
- The US pharmaceutical industry is one of the largest in the world, contributing significantly to the country’s economy. In 2020, the industry’s estimated revenue surpassed USD 534.21 billion, making it a vital sector in the healthcare landscape.
About the Client
- Our recent client is a multinational pharma giant, headquartered in the USA with annual revenue of over $2 billion, specializing in the rare disease market space.
- The client’sobjective was to compare the disease recurrence rate and patient survival rate for 2 different patient groups.
- By unlocking the untapped potential of Clinical Trial Analysis and Digital Medical Affairs, our client aimed to revolutionize the treatment landscape for rare diseases. Their unwavering dedication to improving patient lives served as the driving force behind their pursuit of groundbreaking discoveries.
- With a keen focus on statistical rigor and meticulous data analysis, our client embarked on a captivating journey of exploration and discovery. They aimed to present evidence-backed insights to internal stakeholders and regulatory bodies, solidifying their reputation as pioneers in the rare disease market.
Challenges
- In a remarkable collaboration, our client partnered with over 10 prestigious hospitals worldwide to conduct their research in the rare disease market. However, one of the significant challenges they encountered was the diverse parameter tracking systems implemented by each hospital. This complexity presented a hurdle when it came to comparing and analyzing the results effectively. With each hospital utilizing different tracking systems, data collection and standardization became a formidable task.
- Our client recognized the need to navigate this intricacy to ensure accurate and meaningful comparisons between the two patient groups. To address this challenge, the client wanted to implement a Digital Medical Affairs approach that could empower them to effectively compare and analyze the results, unlocking valuable insights in their quest to enhance the understanding and treatment of rare diseases.
- One of the notable obstacles faced by our client during their research in the rare disease market was the absence of a uniform framework to compare the results of the two different treatments administered to patients. This lack of a standardized framework added complexity to the analysis, as it hindered the direct comparison of the treatment outcomes. Each treatment approach had its own set of evaluation criteria and measurement parameters, making it challenging to draw meaningful conclusions. With the implementation of clinical trial analysis combined with digital medical affairs, the client wanted to develop a unified evaluation framework that can encompass key parameters for assessing treatment efficacy and patient outcomes.
Solutions
- Ensuring the completeness and accuracy of patient data is crucial for any research study, particularly in the pharmaceutical industry, where precise insights can shape treatment strategies and patient outcomes. In the case of a pharmaceutical major, although quality control measures were already in place and performed at regular intervals at the trial centers, they expressed dissatisfaction with the initial analysis results and the reliability of the data obtained.
- The pharmaceutical major recognized that even with routine quality control procedures, there could still be potential gaps and inconsistencies in the data. They understood that relying solely on periodic checks might not be sufficient to guarantee the highest level of data integrity. Recognizing the criticality of precise and reliable data for their research in the rare disease market, our client sought to address the limitations identified in the data quality control processes.
- In their comprehensive clinical trial analysis, the Quantzig team implemented sophisticated parametric hazard models, life tables, and competing risk methods to calculate the patient survival rate and disease recurrence rate across different patient groups. By leveraging advanced statistical techniques and utilizing digital medical affairs strategies, our client aimed to extract valuable insights from the clinical trial data.
- The designed parametric hazard models allowed them to assess the risk factors associated with patient survival, enabling a more nuanced understanding of treatment outcomes. In conjunction with life tables, they could estimate survival probabilities over time, providing a comprehensive view of patient survival rates and their dynamics throughout the trial duration. Additionally, by applying competing risk methods, our client examined the occurrence of other events that could potentially impact patient outcomes, thus accounting for a more accurate assessment of disease recurrence rates. These innovative approaches in clinical trial analysis, supported by digital medical affairs methodologies, empowered them to derive precise and reliable insights from their research.


